Camelid antibodies (VHH) versus monoclonal antibodies:
Complex architecture of monoclonal antibody restrain their clinical and diagnosis development. Consequently many attempts have been made to minimize the size and generate smaller binding antibody fragments. In the begining, many problems originate from the compulsory heterodimerization of a VH with its corresponding VL to maintain a proper antigen binding capacity of the potential antibody, including a low expression yield in microbiological systems, low stability and high aggregation potential. Thus, initial attempts to construct smaller antigen-binding antibody fragments based exclusively on single-domain units have not met with considerable success. However, Nature had already performed similar protein engineering successfully with the Camelidae family and the cartilaginous fish. These particular antibodies found in the Camelidae family (Bactrian camel, dromedary, llama) do not have a light chain (Hamers-Casterman et al, 1993). These antibodies bind their antigen using only the variable domain of the heavy chain (VHH). They and are called sdAb (single-domain antibody), the domain has a size of 2 x 4 nm.
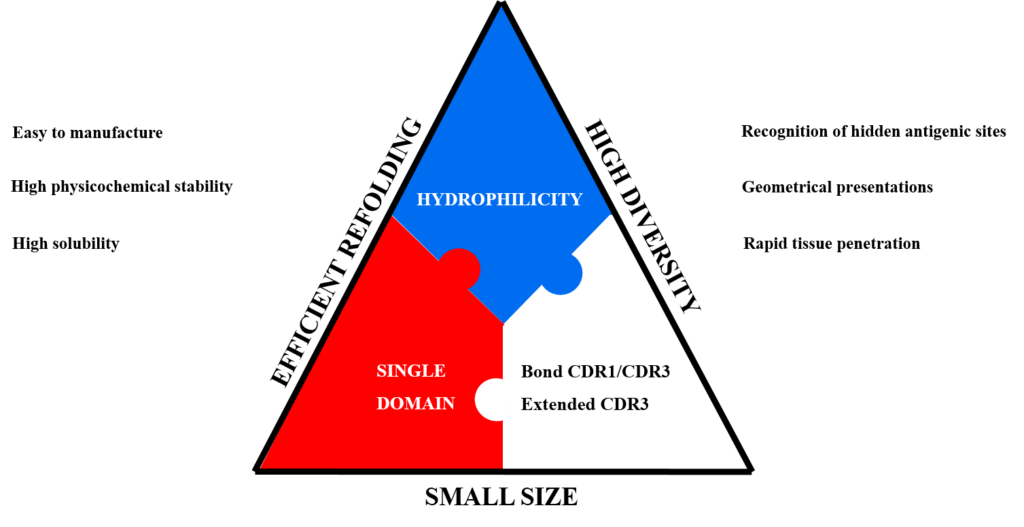
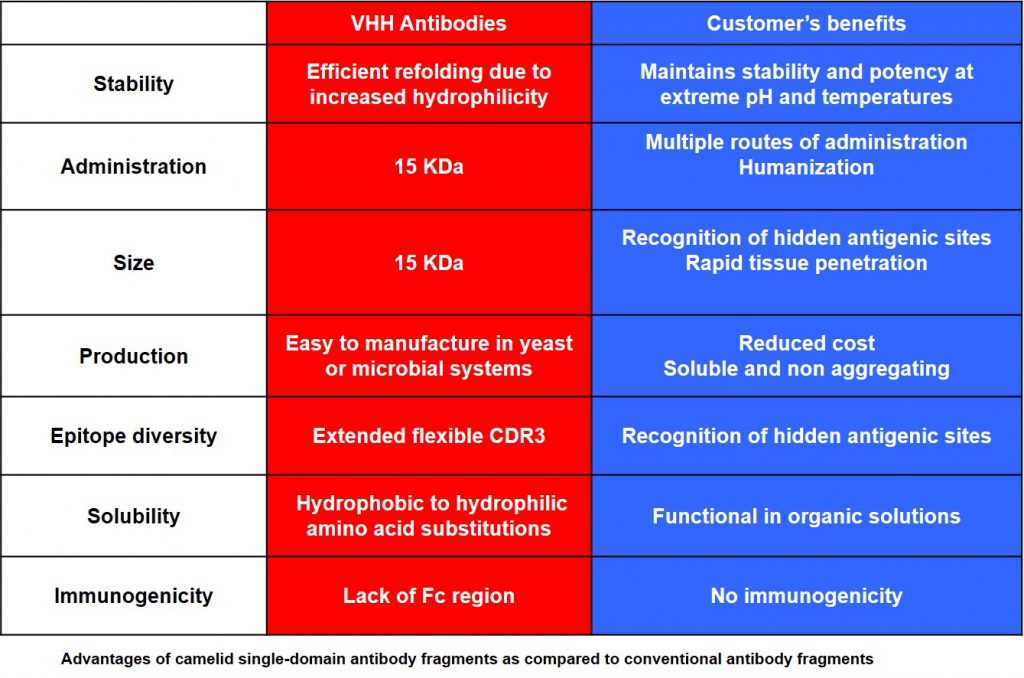
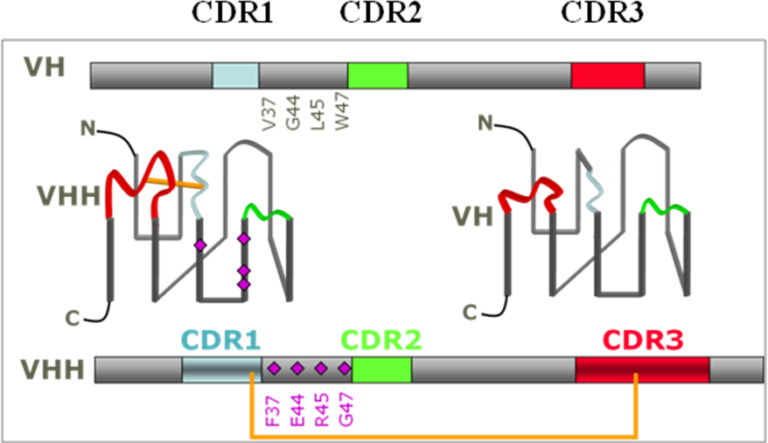
The VHH evolved in camelids in such a way that it already has the advantageous properties that scientists have tried to engineer into the isolated variable domains of classical antibodies to facilitate handling (Huang et al, 2010). These 13 kDa domains generate affinities in the low nanomolar to picomolar range, can easily be mass-produced in bacteria and are shown to be particularly resistant to different physicochemical treatments (Huang et al, 2010). Nanobodies possess structural particularities making them extremely interesting in terms of antigen recognition. As opposed to conventional monoclonal antibodies where the 6 CDRs (“complementarity determining regions”) generally form a cavity that interacts with a protuberant region of the antigen, nanobodies, due to their small antigen binding site (3 variable loops instead of 6) but often with a greater number of amino acids in the CDR3 loop than usually observed in human and murine VH, can penetrate into cavities (Huang et al, 2010) on the antigen surface, often allowing them access to « buried » sites such as those of numerous enzymes (Desmyter et al, 2002),They generally recognize unusual epitopes, inaccessible to conventional antibodies.
VHHs, thanks to their unique properties of size, solubility, stability, low immunogenicity, recognition of uncommon or hidden epitopes, VHHs present significant advantages compared to classical antibodies:
– Solubility: VHHs are naturally soluble in aqueous solution and do not have a tendency to aggregate, due to the substitution of hydrophobic by hydrophilic residues in the framework-2 region compared to conventional antibodies.
– Stability: VHHs are highly heat-stable. Indeed, VHH retain more than 80% of their binding activity after 1 week of incubation at 37°C. They are also stable when exposed to the denaturing effect of chaotropic agents and in the presence of proteases or extreme pH values.
– Expression: VHHs are expressed from a single gene requiring no post-translational modifications. The production process is scalable and expression systems other than bacteria can be used. There is no need of costly mammalian system for VHH expression.
– Immunogenicity: VHH antibodies have many important features, such as their low inherent toxicity, that make theme valuable for biotechnological and medical applications.
– Affinity: the affinity of VHHs is evaluated in one digit nanomolar range in their monovalent form. In multimeric forms, affinity can frequently be reached to picomolar.
– Functionality: VHHs can bind into cavities or active sites of enzyme targets. Other important features are ease and speed of drug discovery and ease of manufacture.